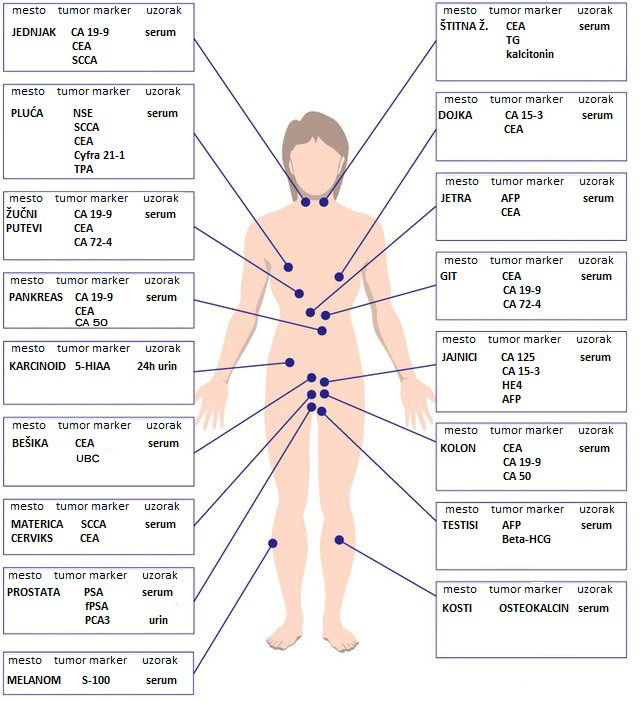
Early serum tumor marker levels after fourteen days of tyrosine kinase inhibitor targeted therapy predicts outcomes in patients with advanced lung adenocarcinoma | PLOS ONE

CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial) -
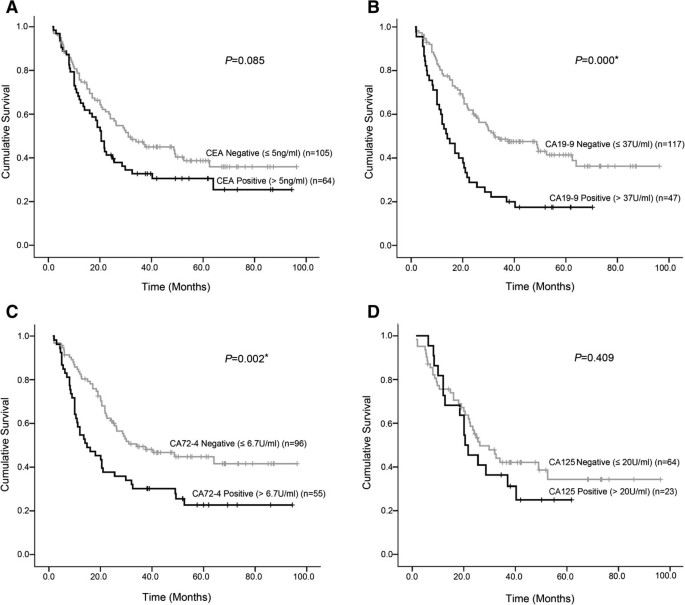
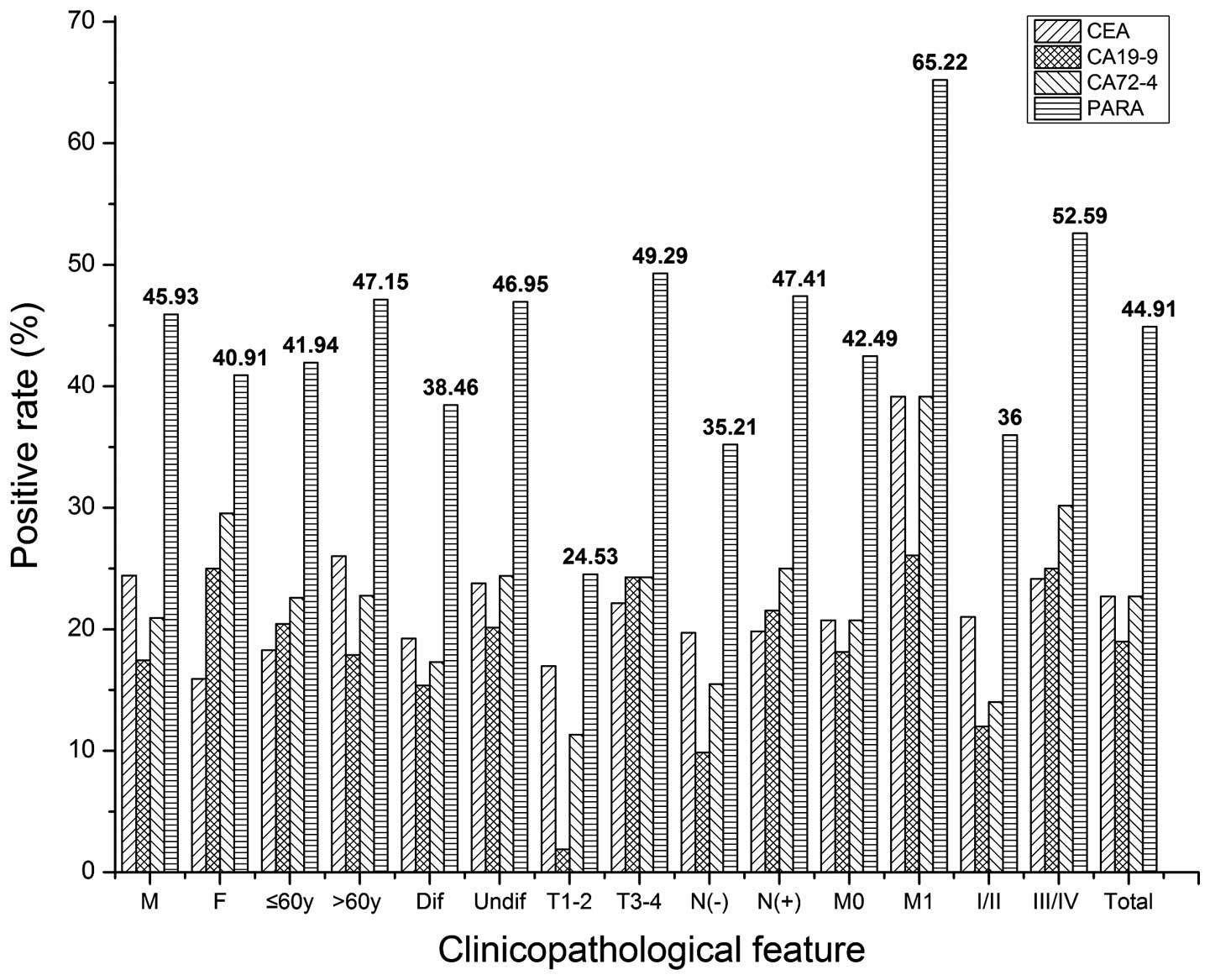
Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy | World Journal of Surgical Oncology | Full Text

CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial) -

CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial) -

Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy | World Journal of Surgical Oncology | Full Text

CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial) -

CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial) -

:max_bytes(150000):strip_icc()/iStock-522878232-59bc10fa054ad90011d8698d.jpg)




:max_bytes(150000):strip_icc()/GettyImages-1219696566-09fde25d71724141929edce6a4881428.jpg)
:max_bytes(150000):strip_icc()/GettyImages-1600186001-5717a1453df78c3fa2232c91.jpg)
:max_bytes(150000):strip_icc()/microscopicpathsquamous-56a5c4cd3df78cf77289d794.jpg)