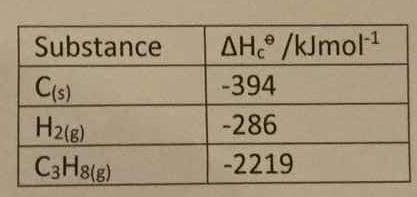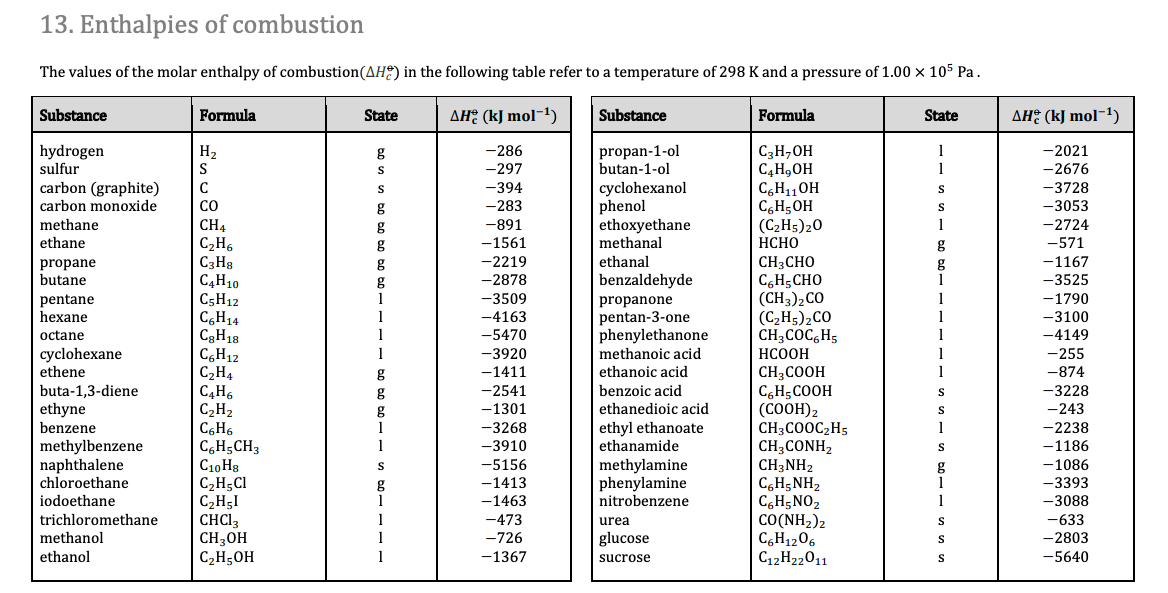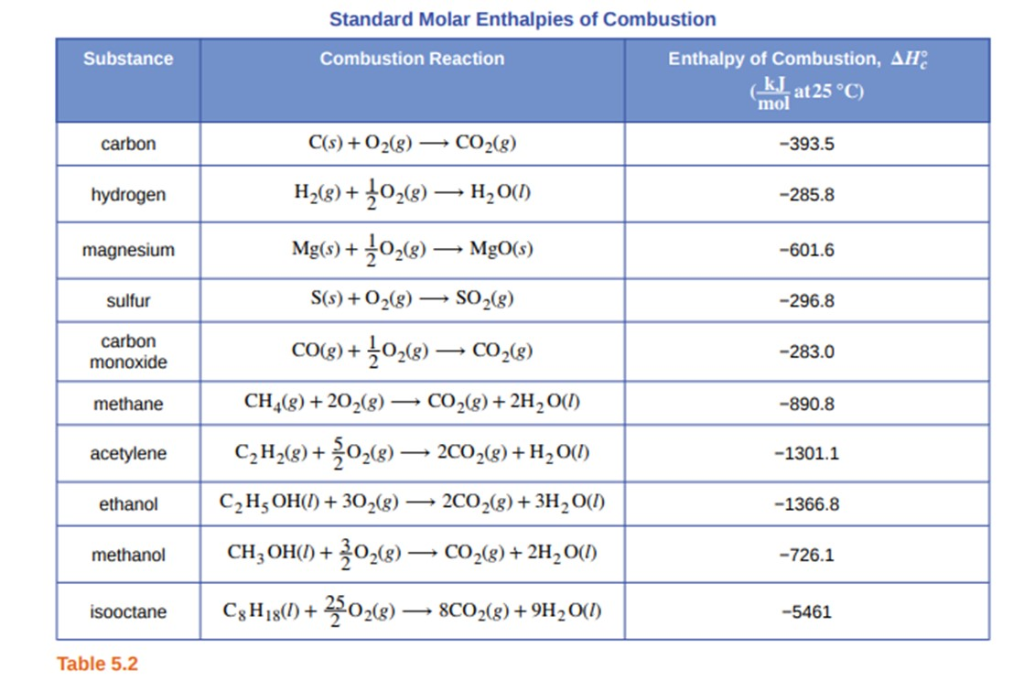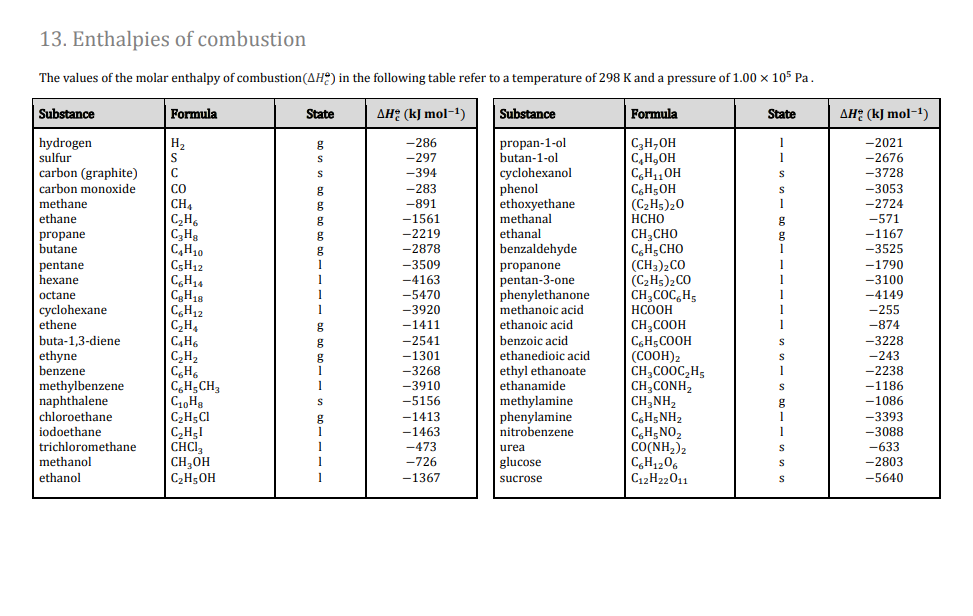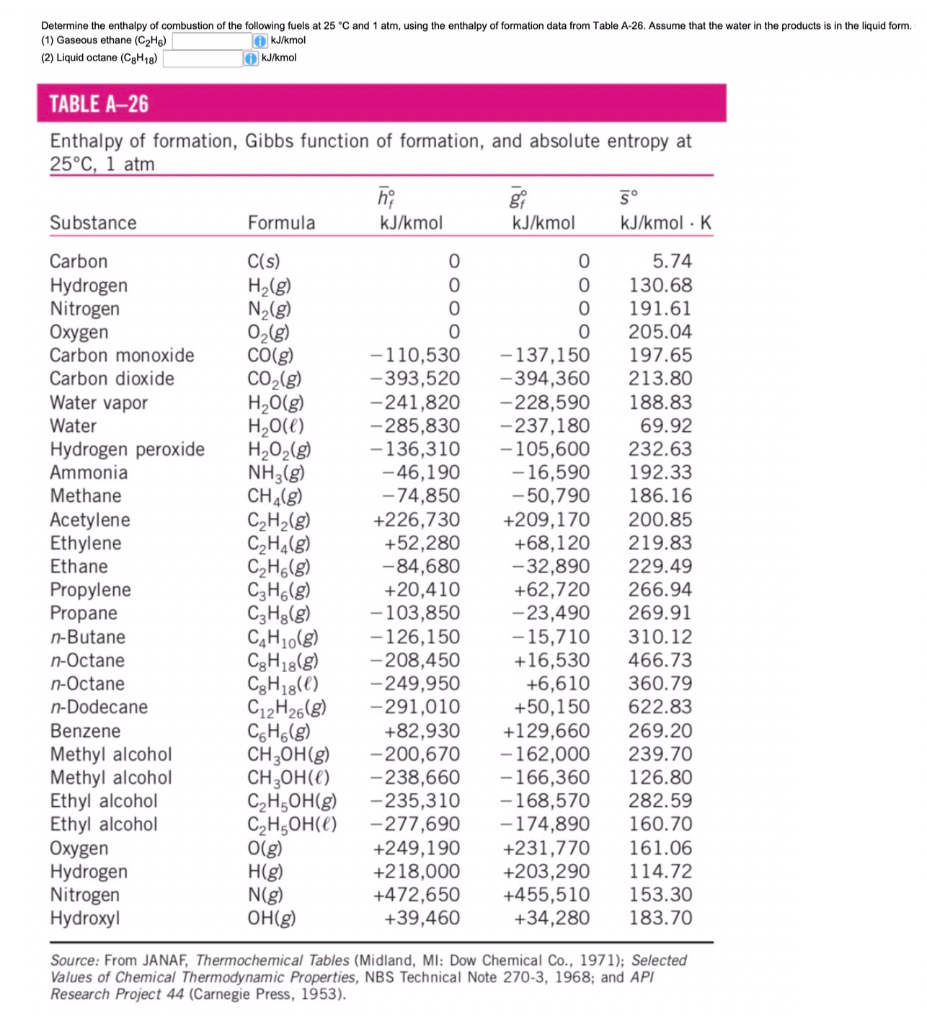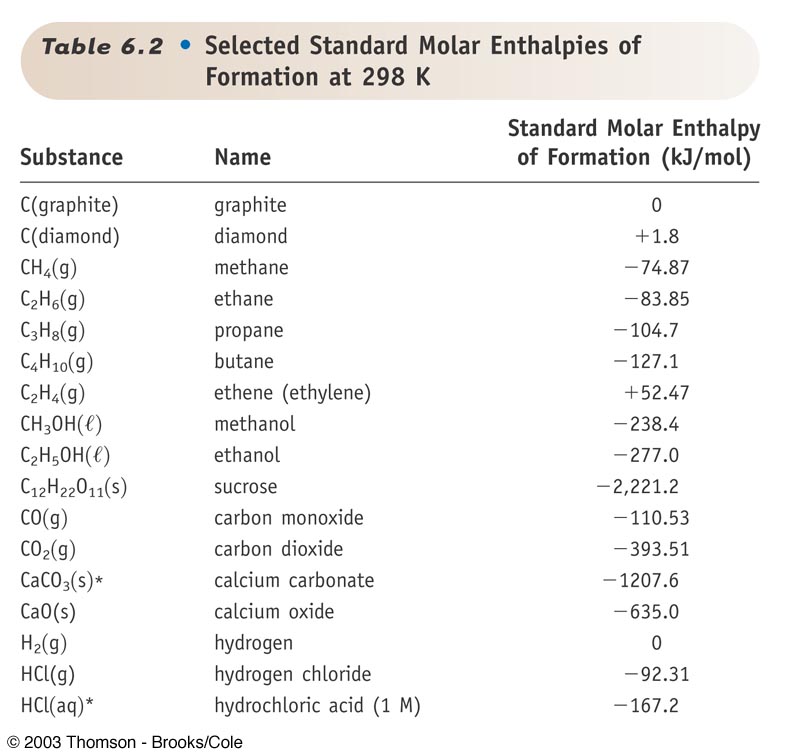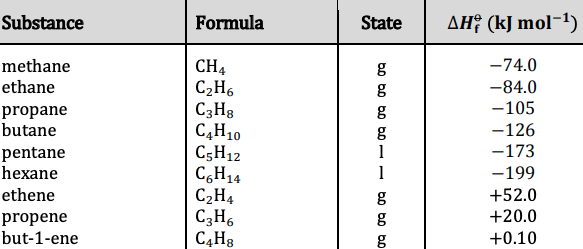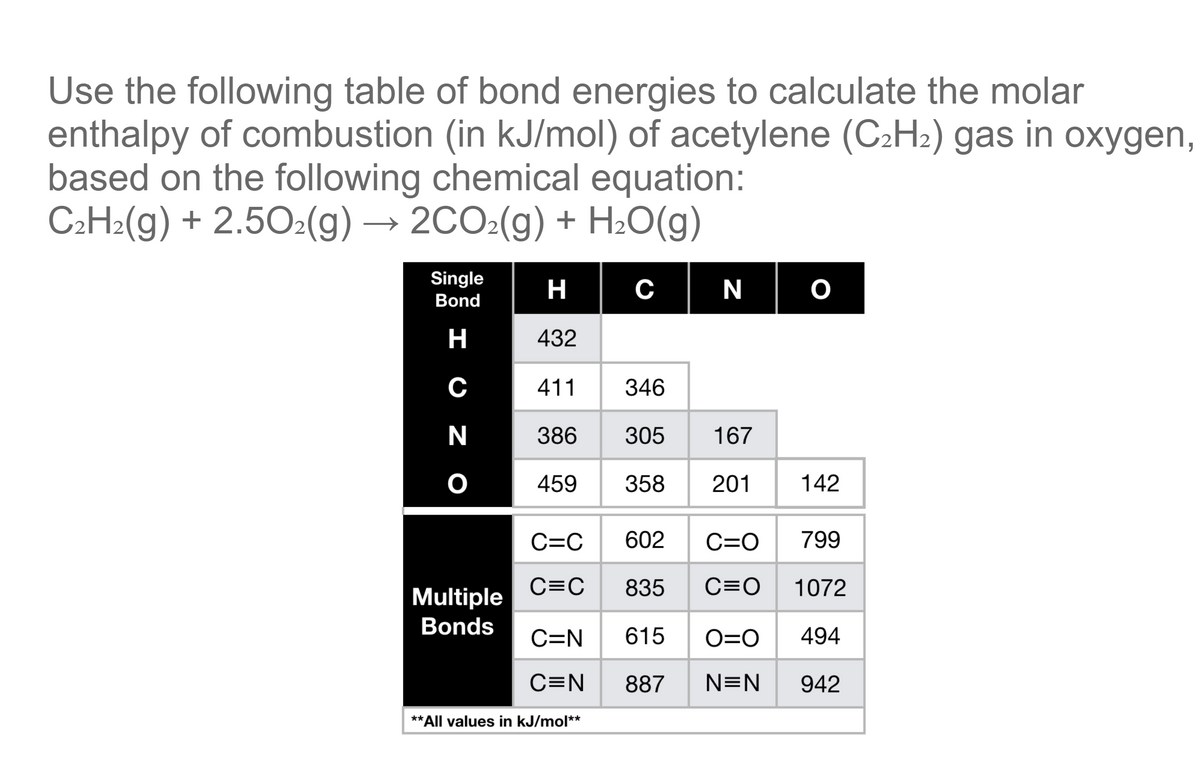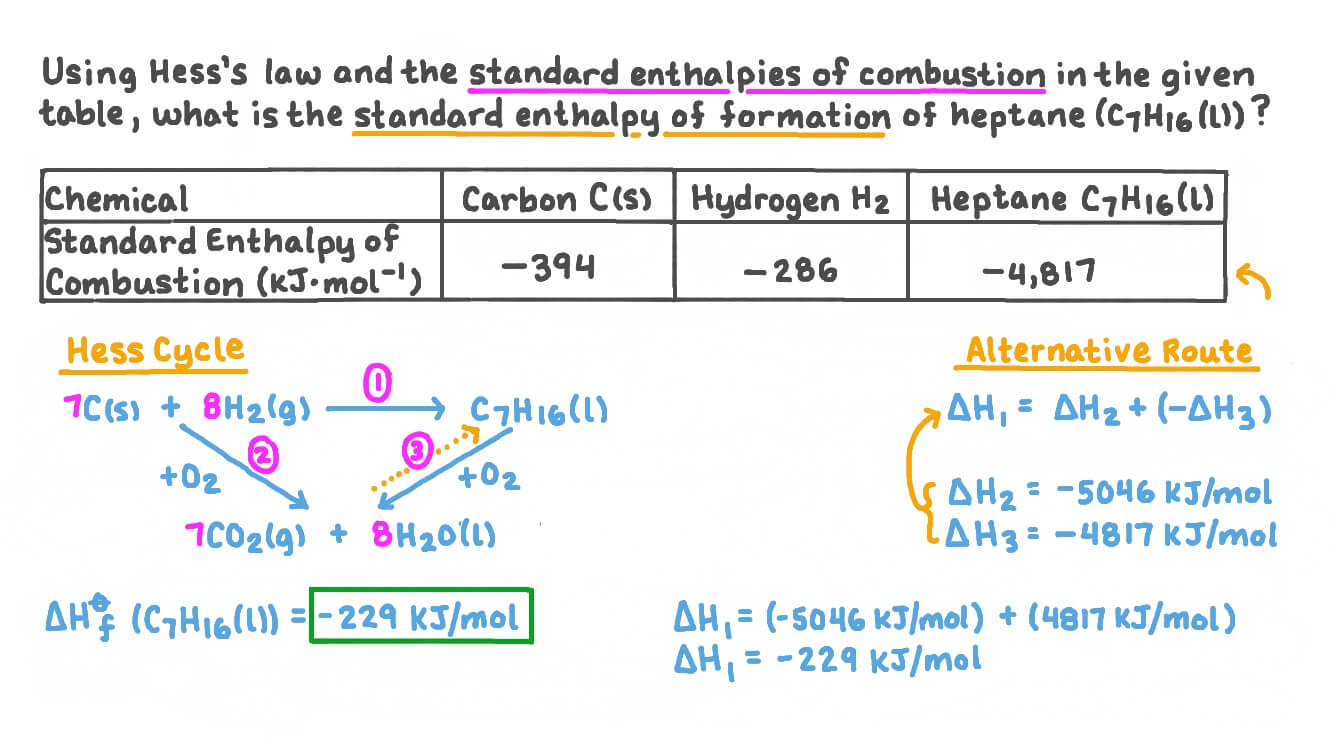
Question Video: Calculating the Standard Enthalpy of Formation for Heptane Using Standard Enthalpies of Combustion | Nagwa
![PDF] Determination of hydrogen content, gross heat of combustion, and net heat of combustion of diesel fuel using FTIR spectroscopy and multivariate calibration | Semantic Scholar PDF] Determination of hydrogen content, gross heat of combustion, and net heat of combustion of diesel fuel using FTIR spectroscopy and multivariate calibration | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/db247bf65cb728be509698d2d946164cf15d3a69/5-Table2-1.png)
PDF] Determination of hydrogen content, gross heat of combustion, and net heat of combustion of diesel fuel using FTIR spectroscopy and multivariate calibration | Semantic Scholar

H |HC = |H |HC + H - H→ H - |H |HC - |H |HC - H From the following bond energies: H - H bond energy : 431.37 kJ mol^-1