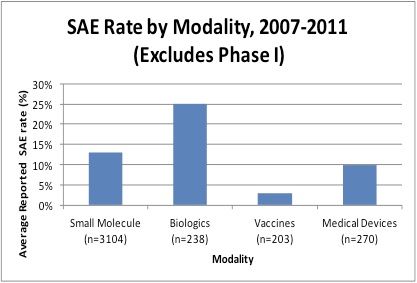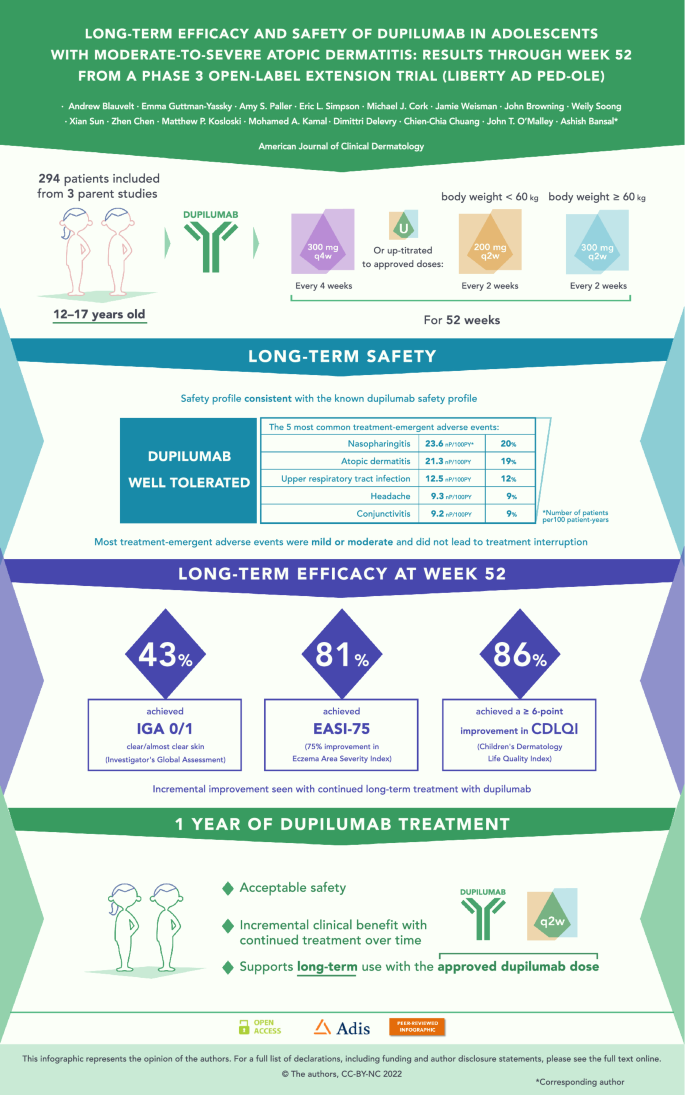
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink

Asenapine for the Acute Treatment of Pediatric Manic or Mixed Episode of Bipolar I Disorder - Journal of the American Academy of Child & Adolescent Psychiatry

A Phase 3, Multicenter, Randomized, Controlled Trial to Evaluate Immune Equivalence and Safety of Multidose and Single-dose Formulations of Vi-DT Typhoid Conjugate Vaccine in Healthy Filipino Individuals 6 Months to 45 Years

Association of Total Medication Burden With Intensive and Standard Blood Pressure Control and Clinical Outcomes: A Secondary Analysis of SPRINT | Hypertension
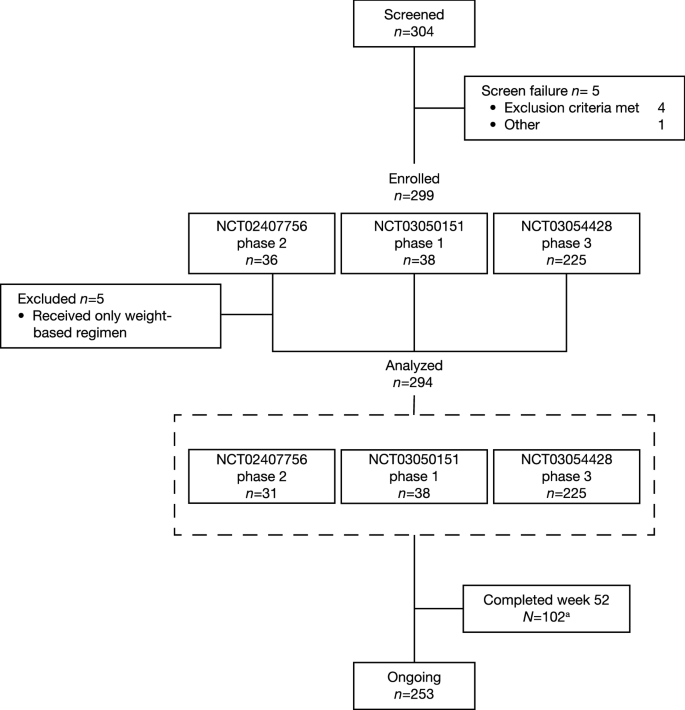
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink

Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial | Annals of the Rheumatic Diseases
Central adjudication of serious adverse events did not affect trial's safety results: Data from the Efficacy of Nitric Oxide in Stroke (ENOS) trial | PLOS ONE
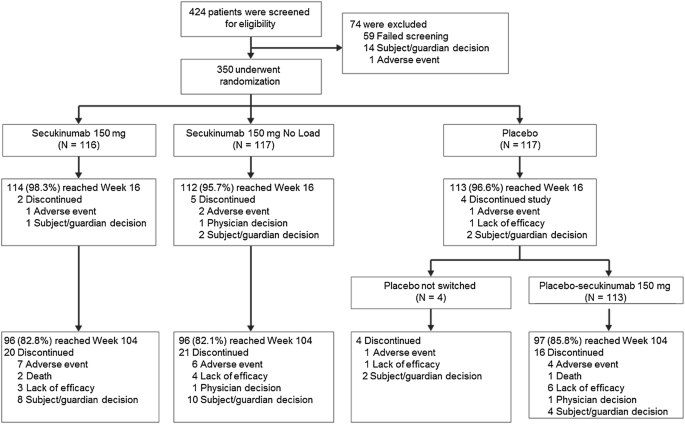
Efficacy and Safety of Secukinumab 150 mg with and Without Loading Regimen in Ankylosing Spondylitis: 104-week Results from MEASURE 4 Study | SpringerLink
Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-c

The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment - ScienceDirect

The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment - ScienceDirect

Fatty Acid Metabolites Combine with Reduced β Oxidation to Activate Th17 Inflammation in Human Type 2 Diabetes - ScienceDirect
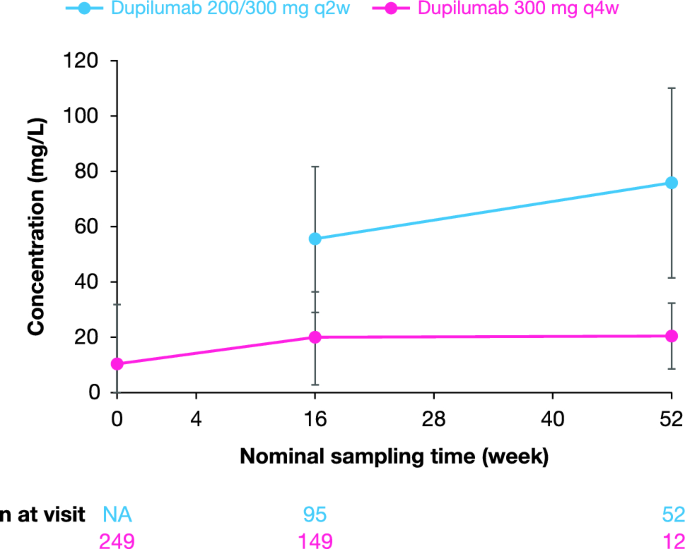
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink

Safety and immunogenicity of Vi-DT conjugate vaccine among 6-23-month-old children: Phase II, randomized, dose-scheduling, observer-blind Study - eClinicalMedicine

Dupilumab provides favourable long‐term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open‐label phase IIa study and subsequent phase III

A Phase 3, Multicenter, Randomized, Controlled Trial to Evaluate Immune Equivalence and Safety of Multidose and Single-dose Formulations of Vi-DT Typhoid Conjugate Vaccine in Healthy Filipino Individuals 6 Months to 45 Years
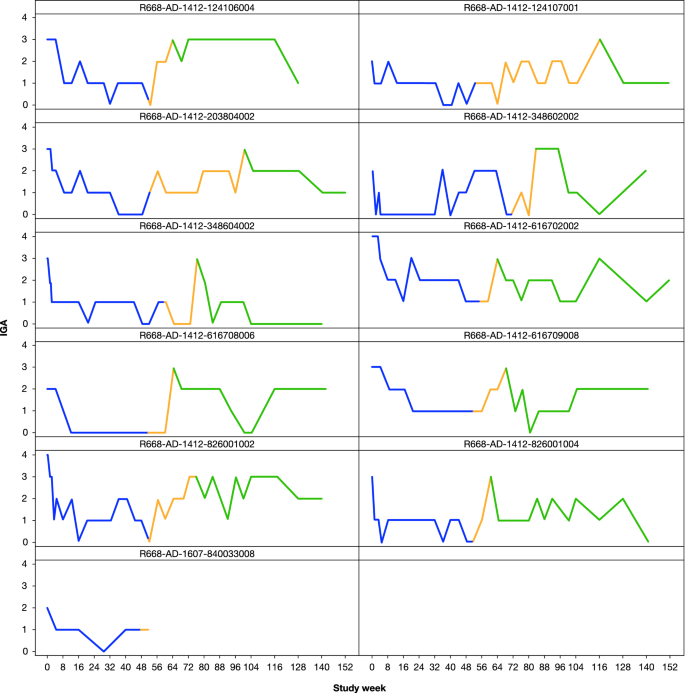
Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE) | SpringerLink
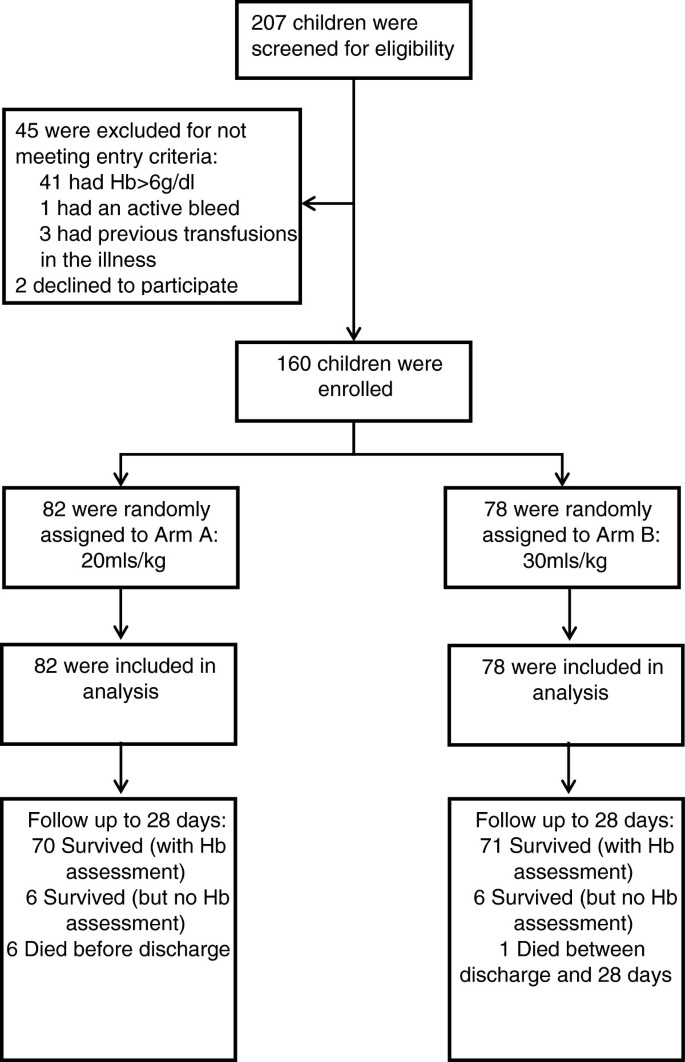
Phase II trial of standard versus increased transfusion volume in Ugandan children with acute severe anemia | BMC Medicine | Full Text
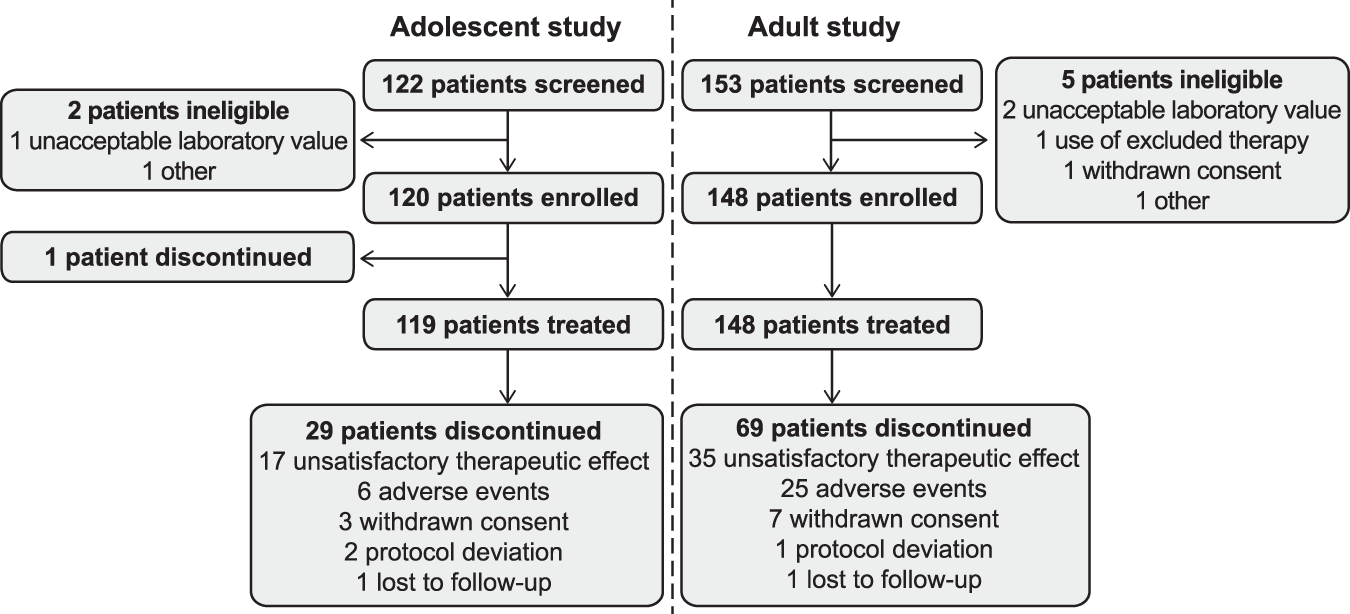
Mavoglurant in Fragile X Syndrome: Results of two open-label, extension trials in adults and adolescents | Scientific Reports
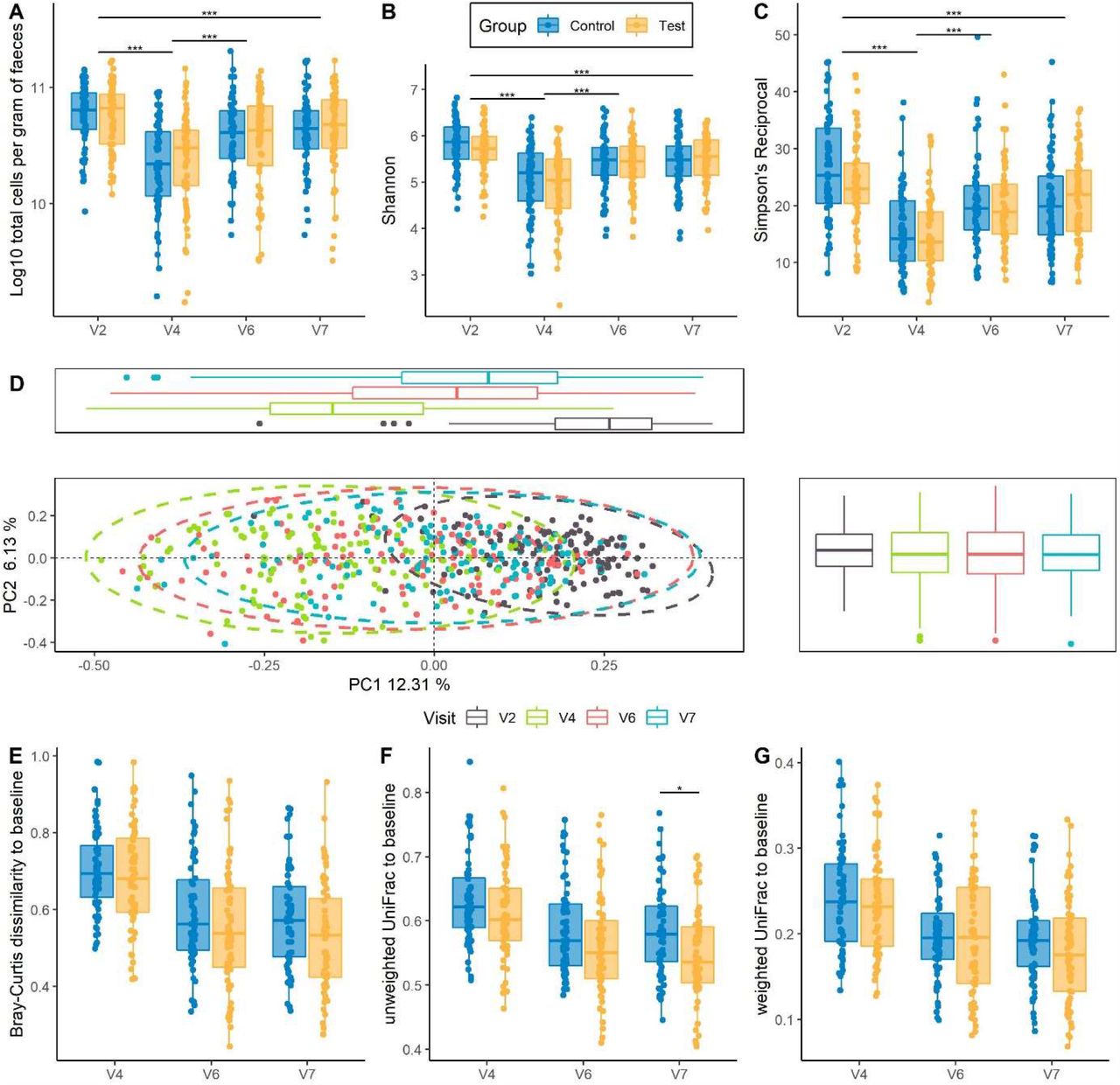
Multi-strain fermented milk promotes gut microbiota recovery after Helicobacter pylori therapy: a randomised, controlled trial | medRxiv

The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment - ScienceDirect

Enrollment and outcomes for Stage2. Trial schedule was similar to... | Download High-Resolution Scientific Diagram